Scientific and medical communities have called the shortage of investigators a crisis that will impact far more than the 50 million Americans currently affected by…
Child Neurology Foundation is conducting a survey concerning knowledge and communication about epilepsy between medical professionals and families. Results will be used to improve conversations…
First antiepileptic drug (AED) to apply FDA’s regulatory pathway of extrapolation for monotherapy use; FDA proposed using this pathway to get monotherapy options to…
Our partners at the Tuberous Sclerosis Alliance are now offering their 3rd annual TS Alliance Junior Leader Program. If you are a young adult affected…
Each year, CNF selects a topic to be the focus of its child neurology community educational initiative. For 2017, CNF selected Sudden Unexplained Death in Epilepsy (SUDEP)…
CCPM is on the Move Children’s Cerebral Palsy Movement (CCPM) executed a successful multi-institutional, multi-disciplinary scientific pilot study, involving boys & girls with CP that…
Led by CNF, the consensus statement, The Neurologist’s Role in Supporting Transition to Adult Health Care* was published in July 2016. The statement identifies 8 Common Principles for…
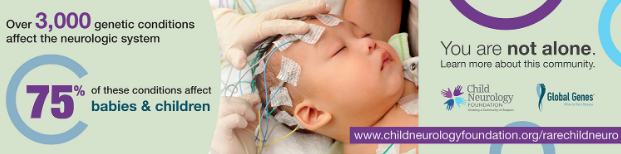
Children and their families who live with rare conditions often feel isolated. We want to change isolation to empowerment. The CNF/Global Genes RARE Child Neurology…
Every year, 1 in 150 people who have uncontrolled seizures dies from Sudden Unexpected Death in Epilepsy (SUDEP). It is the leading epilepsy-related cause of…
CNF offers Grants & Scholarships every year to families in our child neurology community, including the RISE Family grants to help during COVID-19.
Use the forms in this free Notebook to tell the respite caregivers about your child and his/her needs.
CAMBRIDGE, England, April 26, 2017 /PRNewswire/ — Proximagen Limited (Proximagen) today announced that its pivotal Phase 3 trial of intranasal midazolam (USL261) for the rescue treatment…
FDA Approves Expanded Indication for Qudexy® XR (topiramate) Extended-Release Capsules to Include Prophylaxis of Migraine Headache in Adults and Adolescents Molecule Most Prescribed by Neurologists…
Neurology Reviews presents its third annual Rare Neurological Disease Special Report, a compendium of articles and information covering a wide range of rare neurologic diseases. CNF…
MEDIA RELEASE • COMMUNIQUE AUX MEDIAS Novartis International AG Novartis Global Communications CH-4002 Basel Switzerland https://www.novartis.com • MEDIENMITTEILUNG Novartis drug Votubia® receives EU approval to…
Learn important facts about creatine deficiencies by watching this video produced by the Association for Creatine Deficiencies (ACD, creatineinfo.org).
Basel, January 31, 2017 – Novartis today announced that the European Commission has approved Votubia® (everolimus) dispersible tablets* as an adjunctive treatment for patients aged…
The 121st Fort Worth Stock Show offers fun and entertainment for lots of fans. However for more than 200 horseback riders with special needs the…